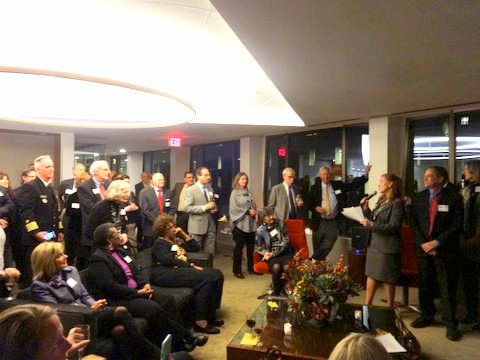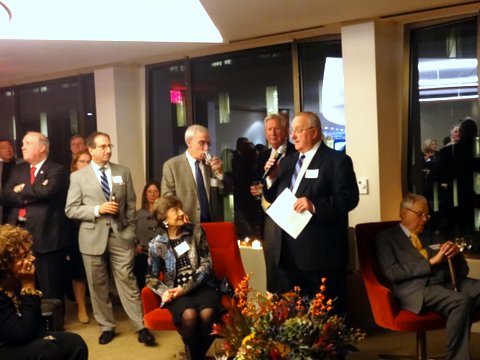- This event has passed.
FDAAA Holds End of Year Reception as a “Tribute to Center Directors – Past and Present”
November 18, 2014

About 100 members of the FDAAA gathered on Tuesday November 18 in downtown Washington to recognize and honor former and current directors of FDA centers. Fourteen center directors attended.
Nancy Myers, FDAAA chairperson, who MCed the event, noted the important role that center directors play at FDA, by providing leadership and direction for programs including product approvals and compliance. Ed Steele, FDAAA president read the following remarks from Commissioner Margaret Hamburg who was unable to attend in person:
I am sorry that I could not attend the tribute to FDA’s Center directors past and present, due to my long planned trip to China. However, I want to pass along my personal appreciation for the contribution our Center directors have made and continue to make to our nation’s health and well-being. Before becoming FDA’s Commissioner, I had a broad, but incomplete appreciation – like that of many Americans – for FDA’s role in what is often called out consumer product “safety net.” My five-plus years at FDA have given me a much fuller understanding of how remarkable FDA is as an institution that protects all of us every single day. And the range of roles and responsibilities that the Center directors have taken on over the years is nothing short of extraordinary. While the FDA is comprised of thousands of truly dedicated people, it is the Center Directors that provide the critical leadership their employees need to realize those exceptional achievements. You are all singular leaders and my respect for your accomplishments is boundless. You are genuinely awe inspiring. Thank you so much for all you have done year after year and for being – in so many ways – the heart and soul of the Food and Drug Administration.
The gathering was held at FoxKiser, a law firm in downtown DC. Sponsors of the event included FoxKiser, Biogen Idec, Greenleaf Health, Hogan Lovells, Keller & Heckman, King and Spalding, Alston & Byrd, Catalyst Healthcare Consulting and, Biotech Policy Group.
The event is part of a series sponsored by the FDAAA to recognize leaders at the FDA and to provide an opportunity for FDAAA members to gather in an informal setting with friends and former colleagues.